Section Branding
Header Content
More Of The Antiviral Drug Remdesivir Shipping To Georgia Hospitals
Primary Content
A second shipment of the antiviral drug remdesivir is heading to 29 Navicent, Northside, Piedmont and WellStar hospitals, the state health department said Thursday.
While the first round of 30 cases allotted from the federal government could treat about 110 patients, depending on how long each patient needs treatment, this shipment will treat more than 300 patients, the Georgia Department of Public Health said in a statement.
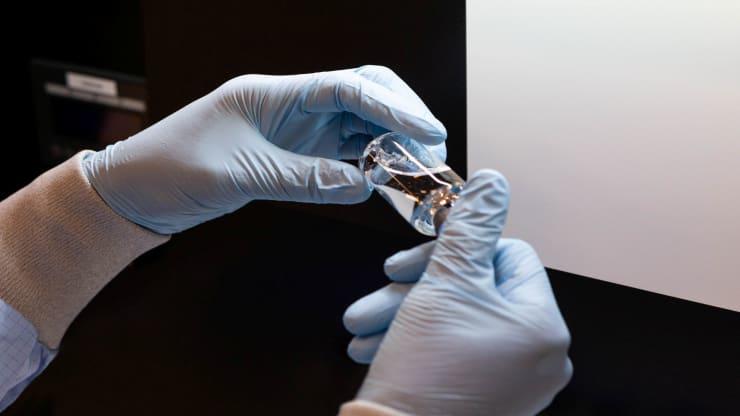
Remdesivir is an antiviral medicine being used to treat hospitalized patients with serious symptoms caused by COVID-19 like low oxygen levels or pneumonia. It has been found to shorten the duration of the disease in patients being treated in inpatient hospital settings. Remdesivir is given intravenously and decreases the amount of coronavirus in the body, helping patients recover faster.
PREVIOUS COVERAGE:
- Remdesivir 101: What Is This Drug, Are There Alternatives And How Much Might It Cost?
- Antiviral Drug Remdesivir Shows Promise Against COVID-19 In Trials
- Georgia Hospitals Receive Remdesivir To Treat COVID-19 Patients
The patients who will get remdesivir are COVID-19 positive, on a ventilator or currently being treated with extracorporeal membrane oxygenation, a machine that takes over the work of the heart and lungs.
These criteria are subject to change based on the availability of remdesivir and the development of patient care at hospital facilities across the state.
By Friday, state officials expect to have a third allotment that could be used to treat children. Currently, about 1% of Georgia coronavirus cases affect children under age 12. The third allotment of remdesivir will be the drug in powder form and can be mixed for dosing based on a child’s weight, the DPH said.
RELATED: CDC Confirms Link Between Pediatric Inflammatory Syndrome And COVID-19
Gilead Sciences, Inc., the maker of remdesivir, is donating approximately 607,000 vials of the experimental drug to treat an estimated 78,000 hospitalized COVID-19 patients under an emergency use agreement. The donation to the United States is part of 1.5 million vials of remdesivir the company is donating worldwide.
Remdesivir has not been approved by the FDA for widespread use because it is considered investigational and it is still being studied. Remdesivir was originally developed for use against Ebola.
Clinical trials for remdesivir were done in Georgia at Emory University Hospital.