Caption
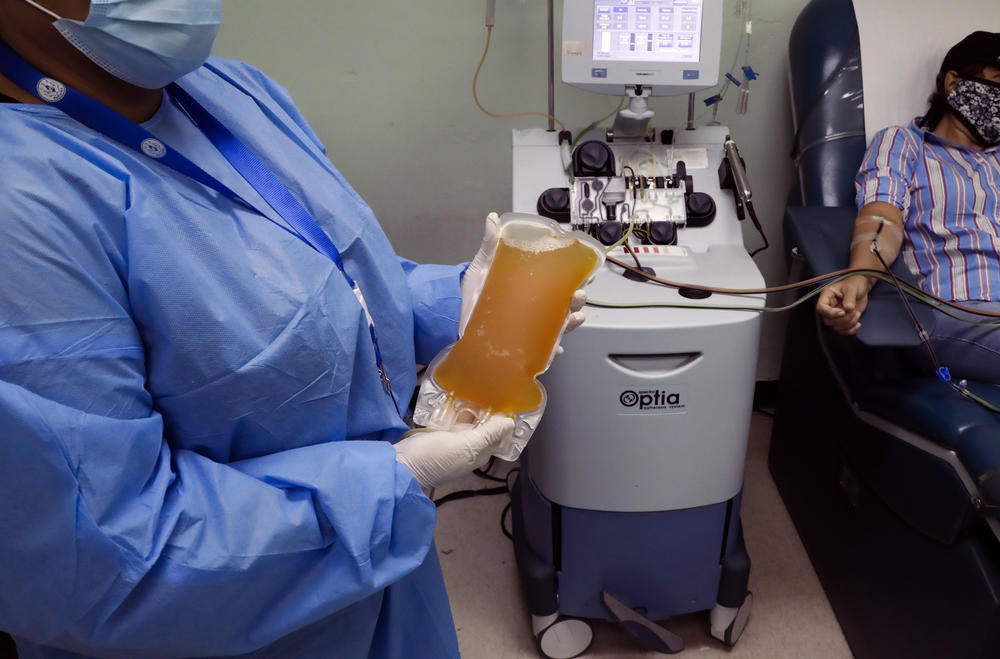
A recovered COVID-19 patient donates blood at the Arnulfo Arias Madrid Hospital, in Panama City, Wednesday, May 13, 2020.
Credit: (AP Photo/Arnulfo Franco)
GPB's "All Things Considered" host Rickey Bevington interviews GPB health care reporter Ellen Eldridge about her recent reporting on convalescent plasma.

A recovered COVID-19 patient donates blood at the Arnulfo Arias Madrid Hospital, in Panama City, Wednesday, May 13, 2020.
As doctors around the world race to find a vaccine for COVID-19, several treatments have been touted, including the antiviral drug remdesivir. Now the U.S. Food and Drug Administration has authorized the emergency use of convalescent plasma.
It's a therapy that uses blood from people who've recovered from an illness to help others. The practice has been around for more than a century. It was even used to combat the Spanish flu in the early 20th Century. Convalescent plasma has not been proven effective in the fight against COVID-19.
GPB's All Things Considered host Rickey Bevington interviews GPB health care reporter Ellen Eldridge about her recent reporting on COVID-19 treatments.
PREVIOUS COVERAGE: COVID-19: Treatment A Political Football Or Miracle? One Survivor's Story.
This conversation has been edited for clarity and conciseness.
Rickey Bevington: What exactly does the use of convalescent plasma on an emergency therapy basis mean for COVID-19 patients?
Ellen Eldridge: Well, the idea is to use the human immune system, which creates antibodies to fight off infection. Anytime somebody is sick, those antibodies remain in the blood plasma after a person heals. So, that plasma from a former, recovered patient is transfused into someone who's actively sick to try to help their immune system kill the virus. Researchers are still looking to see how long those antibodies hang out. In some people, it can be as long as four months. But it's all anecdotal. And the FDA says that while convalescent plasma is safe to try, it is not yet proven effective as a treatment for COVID-19.
Bevington: So, doctors are just trying it right now. But there has been backlash from the medical community concerning even trying this treatment.
Eldridge: That's right. President Trump came out and called the FDA decision to authorize the treatment, the emergency use authorization, a "breakthrough." Part of the problem was timing. It came just before the start of the Republican National Convention. It was controversial to call the treatment a game-changer. And Emory's Dr. Carlos Del Rio immediately held a briefing about the treatment and said that plasma is not a breakthrough.
Some recent studies out of the Mayo Clinic have shown that it's safe to try. But again, not yet proven effective. And then part of the controversy also involves the idea that with this emergency authorization in place, patients might choose to get the antibodies guaranteed as opposed to enrolling in a clinical trial where they might end up getting placebo. And that's not going to help for sure.
Bevington: So why do doctors think this treatment could work at all?
Eldridge: Well, there is anecdotal evidence and, as we've mentioned, it's been used for more than 100 years. I reported on one woman's story, Lisa Hardin. She's a nurse and she was the first person in South Carolina to test positive with COVID-19.
"They would only release convalescent plasma to people who are critically ill because they knew that if they waited, that people were going to be dying," Hardin said.
This 56-year-old woman, she tested positive for COVID-19 back in April. She was admitted to the E.R. with double pneumonia on a Friday night. She found out Easter Sunday that there was a donor way over in Tennessee, Harriett Whitaker, and this woman was available with her plasma so Hardin received the transfusion of convalescent plasma. She said she felt better immediately.
"That immunity attacked the virus in my body and it killed it," Hardin said. "And by the next morning, I could taste. I could smell. And within 24 hours from the time that I had my transfusion, I was well."
So, again, this is one person's experience and doctors can't say for sure that the plasma was the sole factor in Hardin's recovery.
Bevington: So what happens now with the treatment and how will we know whether it's actually effective for the general public?
Eldridge: Other than the anecdotal evidence, it will be tough to prove the science behind this treatment. It's something that's been used a lot. It was even used for Ebola. And I spoke with medical directors at two different plasma clinics. They both said that they are skeptical about the science, but they're hopeful. The treatment is another tool in their kit.
Dr. Matt McClain is a radiologist based in Rome, Georgia. He was an investigator for the Mayo Clinic trial that showed that the treatment is safe for COVID-19 patients.
"This was always meant to be a stopgap measure, sort of a practical protocol that would allow consideration of safety," McClain said. "And then that would be a bridge to an emergency authorization and hopefully randomized trials."
So, again, the clinical trials are still needed to prove efficacy. Right now, there are more than 100 studies around the world looking at the possibility of whether this plasma treatment is going to be effective for COVID-19 patients.
Bevington: Ellen, after this conversation with you, I feel like a lot of Americans might say they're experiencing a kind of whiplash, wondering who to believe about the science of COVID-19. What is the public to do with these conflicting messages?
Eldridge: Sure, yeah. Since we don't have a cure, the best thing is to try to aim for prevention. Avoid getting the coronavirus; avoid getting sick. Scientists simply don't know the reasons why some people get more sick than others. So the best thing to do right now is just to wear a mask when you have to go out in public and wash your hands.