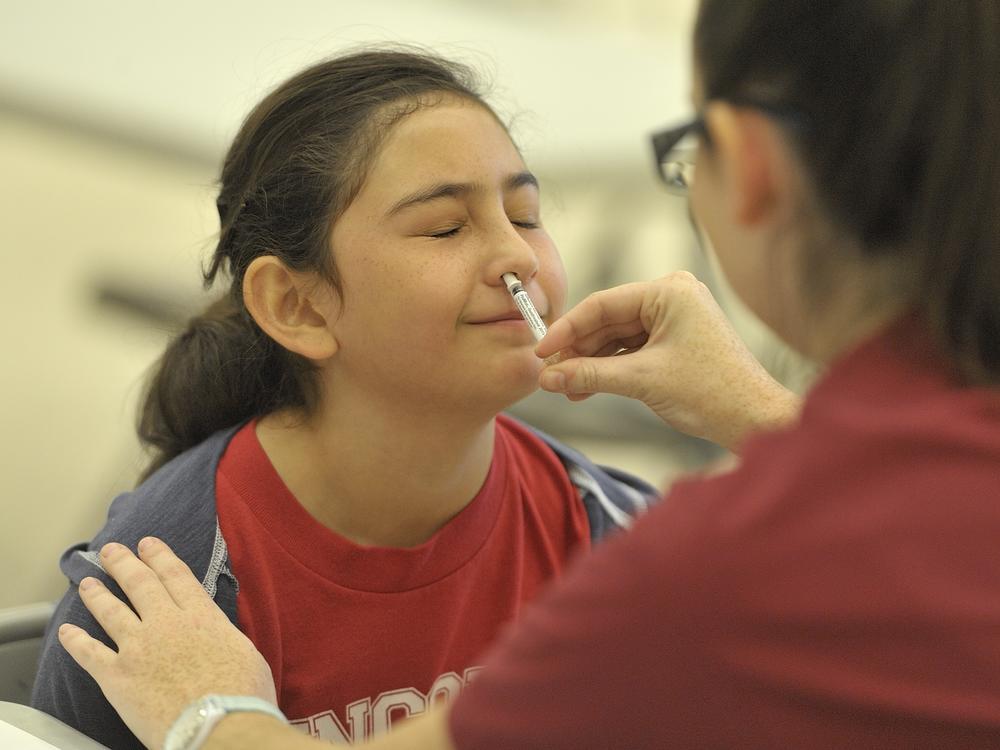Section Branding
Header Content
No needles required: The FDA approves an at-home flu vaccine
Primary Content
The Food and Drug Administration has approved the first flu vaccine that people can administer to themselves at home.
The agency on Friday gave the green light for people who have been screened to give themselves the FluMist nasal spray, which can be ordered directly from an online pharmacy, skipping the need to visit a doctor’s office.
It will still require a prescription from a doctor's office, however. It's expected to be available next year.
FluMist itself is not new — the live attenuated influenza vaccine has had FDA approval for more than two decades. But the ability for adults to order the vaccine at home to administer to people ages 2 to 49 is a breakthrough in convenience and access to preventative care.
“Getting vaccinated each year is the best way to prevent influenza, which causes illness in a substantial proportion of the U.S. population every year and may result in serious complications, including hospitalization and death,” said Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research.
“This approval adds another option for vaccination against influenza disease and demonstrates the FDA’s commitment to advancing public health,” Marks added.
In addition to providing the convenience to get the vaccine delivered right to your door, the nasal spray option could encourage more people who have fears of doctors or needles to inoculate themselves against the flu.
The FluMist nasal spray will be made available through a third-party online pharmacy, where people will complete a screening process to check eligibility. The FDA does recommend that a caregiver administer the spray to children between the ages of 2 and 17.