Section Branding
Header Content
Virtually ouch-free: Promising early data on a measles vaccine delivered via sticker
Primary Content
Vaccine experts tend to be a serious bunch, but many are downright giddy about vaccine clinical trial results presented last week at a medical conference in Seattle.
The actual vaccine isn't new — it's the one used to protect against measles and rubella (German measles) and was formulated decades ago. But new results show that the novel delivery system, in development for more than two decades, could be a big step forward, especially for low-income countries.
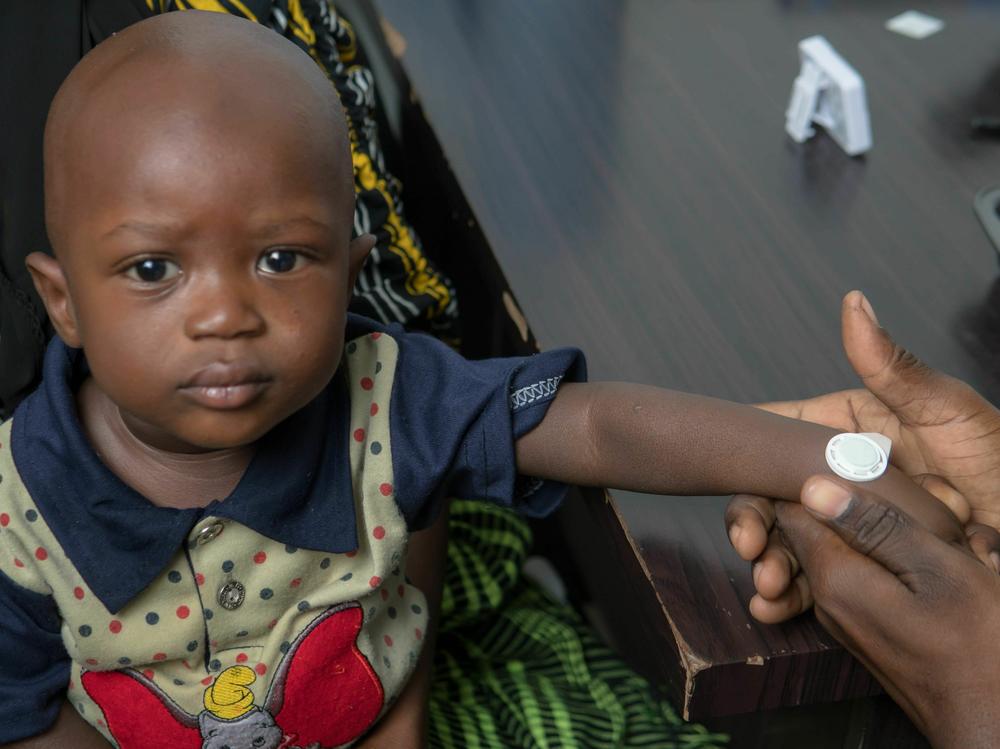
There's no syringe involved. Rather, there's a small adhesive patch — think Band-Aid — containing tiny microarray "needles" made from the vaccine in dry form, says Steve Damon, CEO of Micron Biomedical, which has been working on the patch for about six years.
The patch is a white plastic disk about the size of a quarter. When it's gently pressed onto the patient's wrist, within just a few minutes, the tiny needles deliver the vaccine dose.
The patch can be administered by a lay person with minimal training, says Damon: "The vaccine is pushed into the skin with your thumb or finger to provide the same dose as an injection would but without the involvement of a needle and syringe." Damon says the microneedles penetrate just the outer layer of the skin above the pain receptors, so there is hardly any discomfort. (James Goodson, senior scientist and epidemiologist in the Global Immunization Division at the Centers for Disease Control and Prevention and co-investigator for the study, likens the feeling to Velcro on skin.)
The clinical trial, conducted in The Gambia, was the first to test both the efficacy and safety of the vaccine patch. It included 45 adults, 120 toddlers (15-18 months old) and 120 infants (9-10 months old) who received the measles-rubella vaccine either by the microarray device or by a conventional injection. A month and a half after vaccination, the researchers assessed the immune responses of the trial participants. There were similarly robust responses for both vaccination methods.
In a survey of parents of children enrolled in the trial, the majority said the microarray technology was essentially "painless," and 90% agreed that the patch would be better than an injection for children. The results were presented at a conference on microneedle technology, and according to the company, will soon be published in a peer-reviewed medical journal.
"This is a long overdue technology" says Dr. Gregory Poland, head of the Vaccine Research Group at the Mayo Clinic. Poland was not involved with the clinical trial. "If they can get this down at a price point that makes sense, you don't need a trained health-care worker to administer the vaccine. You eliminate the fear factor, the risk of blood and body fluids from the needle. For the person administering it, you don't have medical waste like you do with a syringe and needle."
The trial was co-led by Ed Clarke, head of infant immunology at Medical Research Council, The Gambia. "These are exciting results which show, for the first time, the potential for microarray patches to safely and effectively deliver vaccines," Clarke said in a statement.
The trial results are important, Dr. Birgitte Giersing, team lead for the WHO's Vaccine Product and Delivery Research Unit, tells NPR in an email. "Alternative vaccine delivery innovations are needed to deliver vaccines to areas that are not easily reached by immunization programs, for example in settings where there are few health facilities, or areas that are difficult to access."
There's another advantage besides ease of delivery. Many vaccines require careful cold storage, which can be difficult or even impossible in some parts of the world. The adhesive patch does not need to be refrigerated. Nor is there a need for clean water to sanitize facilities and supplies — and to dilute the vaccine, which typically comes in a multi-dose concentrated form. The patch would also cut down on wasted vaccine: If only a few children show up on some days with the current system, any remaining vaccine made from concentrate has to be thrown away.
"[For many] reasons we think [the vaccine patch] really has the potential to help us reach more children and to accomplish our mission, which is to save lives," says David Robinson, deputy director, Vaccine Development & Surveillance at the Bill & Melinda Gates Foundation, which helped fund the clinical trial. (Note: The Gates Foundation is a funder of this blog and of NPR.)
According to UNICEF, about a fifth of the world's children aren't fully vaccinated for childhood diseases. The reasons include hesitation by the parents, insufficient vaccine education, expense, and lack of health-care infrastructure. A 2023 report by UNICEF found that the COVID-19 pandemic has been a "disaster" for childhood immunization. The State of the World's Children report found that the pandemic "severely disrupted childhood immunization, with 67 million children missing out entirely or partially on routine immunization between 2019 and 2021."
In addition to microarray patches, global health groups such as GAVI, the Vaccine Alliance, a coalition that includes governments, foundations and NGOs, are funding alternative vaccine delivery systems including nasal, oral and inhaled-by-mouth options that may offer advantages to the mix-and-fill syringe systems currently used for many vaccines.
Measles and rubella aren't the only vaccines being tested in microarray patches. Study co-investigator James Goodson says other candidates include rabies, tuberculosis and hepatitis B. The Measles Rubella partnership, which includes WHO, the Centers for Disease Control and the American Red Cross, helped coordinate research and distribution for Micron Biomedical's patch.
While the early clinical trial news is promising for the measles-rubella microarray patch, scientists say it could take at least five to seven years before it is available for sale. That goal, says Goodson, will require much larger clinical trials and authorization from a country's regulatory agencies — as well as a willingness by vaccine manufacturers to spend money on the technology.
And even the cleverest technology won't clear all the barriers to full immunization for the world's children, says Dr. Derrick Sim, managing director, Vaccine Markets & Health Security at GAVI, the Vaccine Alliance. "[The microarray] technology does solve for quite a number of those barriers, [such as] making sure that the vaccine is more comfortable [potentially reducing] resistance by the parents or the child towards the vaccination," says Sim. What it probably does not address, he says, "is perhaps some of the things like education [and] misinformation. We need to ensure that communities understand the value of vaccines, and that they understand the reason why it's important to vaccinate against measles and rubella."
Meanwhile, the WHO's Birgitte Giersing tells NPR that international organizations are already thinking about how to increase access to these novel vaccine systems. "New vaccine products are likely to cost more than the current standard of care (multi-dose vials and syringes), and this is a potential barrier to uptake," Giersing says in the emailed statement. "In the case of microarray patches, many global partners are working together to quantify how these innovations can save costs at the implementation level, for example by reducing delivery costs, to offset the potentially higher price, and to find novel financing mechanisms to support initial introduction in early adopter countries."
Many things need to fall into place for an immunization effort to succeed – from the economics to logistics to public acceptance. "We are calling on the various actors to crowd in," says Derrick Sim, "to ensure that further investment in clinical trials, regulatory [processes] and a pilot plan will be available, so that we can have development and availability of this vaccine in the future."
Fran Kritz is a health policy reporter based in Washington, D.C., and a regular contributor to NPR. She also reports for the Washington Post and Verywell Health. Find her on Twitter: @fkritz
Copyright 2023 NPR. To see more, visit https://www.npr.org.