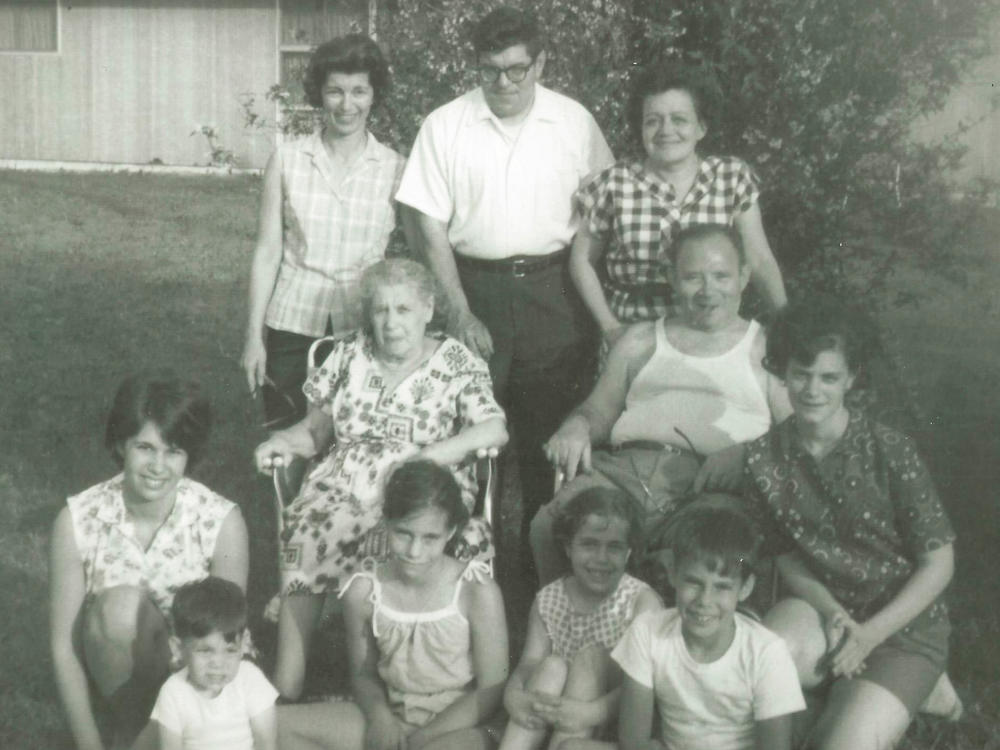Section Branding
Header Content
Why I'm A Vaccine Volunteer: Doing What Needs To Be Done
Primary Content
I come from people who did what needed to be done when faced with personal or community crisis. And I have a long history of experience with vaccines.
So, when I heard about the COVID-19 vaccine trial taking place where I live in Salt Lake City, I didn't hesitate. I already knew the research team because I'd been through two unrelated vaccine trials in the last year. I was familiar with the pin pricks, protocols, clinic visits, informed consent forms and piles of paperwork. I already knew the trial's doctor, nurses and medical assistants.
"Sure," the nurse told me when I called the clinic. "We need people for this trial and you'd be great."
I seemed like a good candidate because I'm at the pandemic-risky age of 66, but relatively healthy and active. My wife Wanda fits that description as well. So, we both endured two hours of questions, medical history reviews, and checks of temperature, blood pressure, hearts and lungs. We scrupulously read informed consent forms, considered all the caveats, did our own research, and signed. Needles and vials came out and we had our first sharp but gentle jabs.
It was a big production that day and later for the second round of injections. The medical assistants, nurse and doctor flitted in and out of the tiny examination room, always politely apologizing for the questions, the probing, the crowding, and especially the paperwork. We were patients 2 and 3 and they were still working out the kinks of the routine. They were checking each other along the way. "Did you have them sign this page?" "Did you tell them about that?" "Did you get their blood?"
We gave lots of blood.
We didn't know then and we still don't know who in our group of volunteers got the real vaccine and who got the placebo. The research team doesn't know. It's a double-blind study. So, even Moderna, the vaccine developer, isn't supposed to know. Only the independent vaccine overseers are supposed to have access to that information.
Like everyone else, Wanda and I have been trying to guess, based on our reactions to the vaccines. But those reactions were minor, so it's hard to say. Moderna has promised that eventually everyone in the placebo group will be offered the vaccine, so we'll find out then.
In any case, we aren't worried. We know that the Moderna vaccine uses synthetic COVID-19 mRNA. There's nothing in it that could actually give us the coronavirus. And in the early preliminary trial with a very small group, there were no serious side effects.
Still, friends asked us why we did this.
That got me thinking about my people and their history, and my own long experience with vaccines.
First, there's the example set by my grandmother. When she boarded the New Rochelle, a massive passenger ship, in Le Havre, France, in 1921, she was 24 and pregnant. Really pregnant — close to delivery. And she feared that alone would get her kicked off the ship before it left port, or deported once she arrived at Ellis Island. She and 22-year-old Moise, the man who would become my grandfather, couldn't again face deadly pogroms, severely restricted lives, and forced service in armies fighting hopeless battles. That's what they and other Jews were fleeing in eastern Europe.
Moise and Ruchel were determined to get to America, their promised land, so Ruchel did what needed to be done. She put on layer after layer of heavy coats, and wore them the entire voyage. It was winter on the high seas so that may have seemed sensible. Other immigrants wore lots of clothes, too, so they had more room for more things in their luggage.
It worked for Ruchel. She hid that pregnancy from the 2,000 other migrants on board, from the ship's crew, and from the immigration officers at Ellis Island.
One officer did note a medical problem on the immigration arrival form, so my family suspects that a government doctor discovered the pregnancy during an examination but let it go — doing what needed to be done for these young refugees desperate for new lives.
Ruchel and Moise Berkes' daughter Reba was born four days later; the new father sold apples on the street to support his suddenly expanded family.
My father Milton came along a few years after that. He eventually became a local elected official in a Philadelphia suburb. In 1957, when I was 3, the first non-white family moved into a Levittown neighborhood, and my father and other community leaders found themselves facing down white racists, who rioted night after night. The rioters burned a cross, honked horns, and screamed ugly insults and threats.
My dad and a group of other leaders stood with the family, as they all stood up to the mob, which required unblinking commitment and some physical and political risk. The rioting subsided, the Black family stayed, and more families of color moved in, too. In his own time of reckoning, my father did what needed to be done.
Later, as a state representative and Pennsylvania's first drug czar, my father Milton authored a law that transformed Pennsylvania's treatment of drug addiction, turning state policy away from incarceration and toward rehabilitation. His work on drug policy resulted in another test — for both of us.
I was in my early teens, home alone one night, forced to field the regular phone calls we'd get from people looking for my dad, often looking for his help. One caller that night was desperate. Suicidal. He talked about his drug use and his hopelessness. "There's no point in going on," he cried. So, I just talked and talked, kid to kid, thinking fast about what to say. "There's help," I told him. "There are people who can get you through this. Hang on. I'll get someone to call you."
My dad phoned the desperate caller back later and he did provide help and hope, connecting him with a treatment program. That night we both did what needed to be done.
There's also this, when I think about why I signed up for experimental shots aimed at COVID-19: I have my own long and deep experience with vaccines, starting at a very early age.
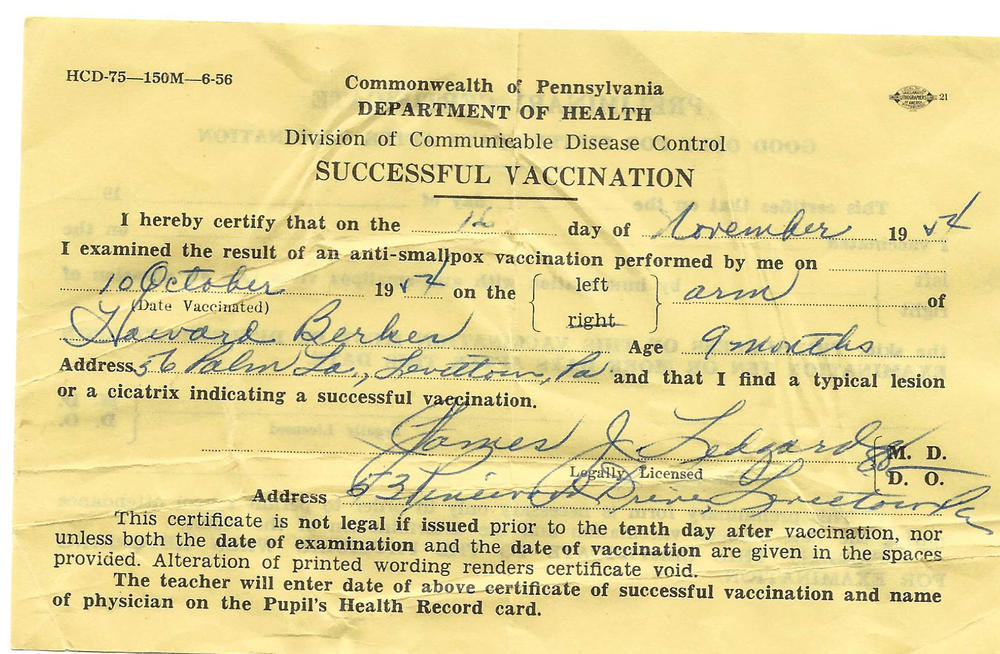
I still have a wrinkled yellow certificate dated November 1954, from the Pennsylvania Department of Health. It proved to all who cared that I had a "successful vaccination" for smallpox in my right arm at age 9 months.
And maybe more influential was my experience three years later. when the United States was panicking about the crippling infectious disease called polio. First came the Salk vaccine, which required a series of three injections. People waited in lines for hours to get their shots, my mom among them.
My mom Ethel saved for decades a tiny newspaper clipping with a brief headline: "Three-Year-Old Overshot."
I was that 3-year-old, and had gone with my mother to the local hospital. She was getting a Salk vaccine polio shot herself, and I was just standing in line alongside her. I'd already had my three shots — my mom made sure the nurse at the front of the line knew that. But in the rush of people getting poked, and with my mom distracted, a doctor suddenly nailed me in the arm with my own fourth shot.
My mom freaked, but, the doctor told her not to worry. The extra vaccination wouldn't hurt me. Think of it as a booster shot, he said. She kept a sharp eye on me as time wore on, but I was fine.
After that, I was all in for vaccines – a dutiful pincushion for shots, when necessary, against diphtheria, tetanus, pertussis, measles, mumps and rubella.
There was one exception in 1976, when I was 22, and an outbreak of swine flu at an Army base in New Jersey triggered fears of a pandemic. No one off the base became infected and the vaccine that was rushed out seemed to be involved in some rare but nasty side effects. That scared me and I wound up avoiding flu shots for the next 40 years, probably at my own peril.
But with advancing age and increased risk from flu I decided it was time to man up. I not only subjected myself to vaccines again, but became a voluntary guinea pig in trials for new flu and pneumonia vaccines.
I suffered no major side effects from either of those vaccines (or any other) and, coincidentally, both trials were handled by the research team at my medical clinic, which is also conducting the Moderna COVID-19 vaccine trial here in Utah.
So, it was easy for me to sign up for the Moderna trial.
And, beneath it all, there's this: I can't make people wear masks. I can't force anybody to maintain safe physical distance. I can't revive the 280,000 Americans who've died. I can't protect the 15 million who've been diagnosed. I can't restore jobs and paychecks. I can't keep people from losing homes. I can't reopen schools, restaurants, gyms and bars. I can't keep ICU's from overflowing with coronavirus patients. I can't magically ease the burden of doctors, nurses and other medical professionals who are risking their lives every day, and sometimes falling ill and dying themselves.
And I can't force politicians to exercise leadership, and to ignore the selfish political "don't tread on me" resistance to doing what needs to be done.
But I can be Patient 3, tolerating two needle pricks in the arm in a month, giving up vial after vial of blood, enduring multiple deep swabs into my nostril and throat, and regularly reporting any changes in my medications or health.
These are actually very small acts with negligible risk. But, right here and right now, it's precisely what needs to be done.
Howard Berkes is a retired NPR Investigations Correspondent living in Salt Lake City. He spent 38 years at NPR and has earned more than 40 national journalism awards.
Copyright 2020 NPR. To see more, visit https://www.npr.org.