Section Branding
Header Content
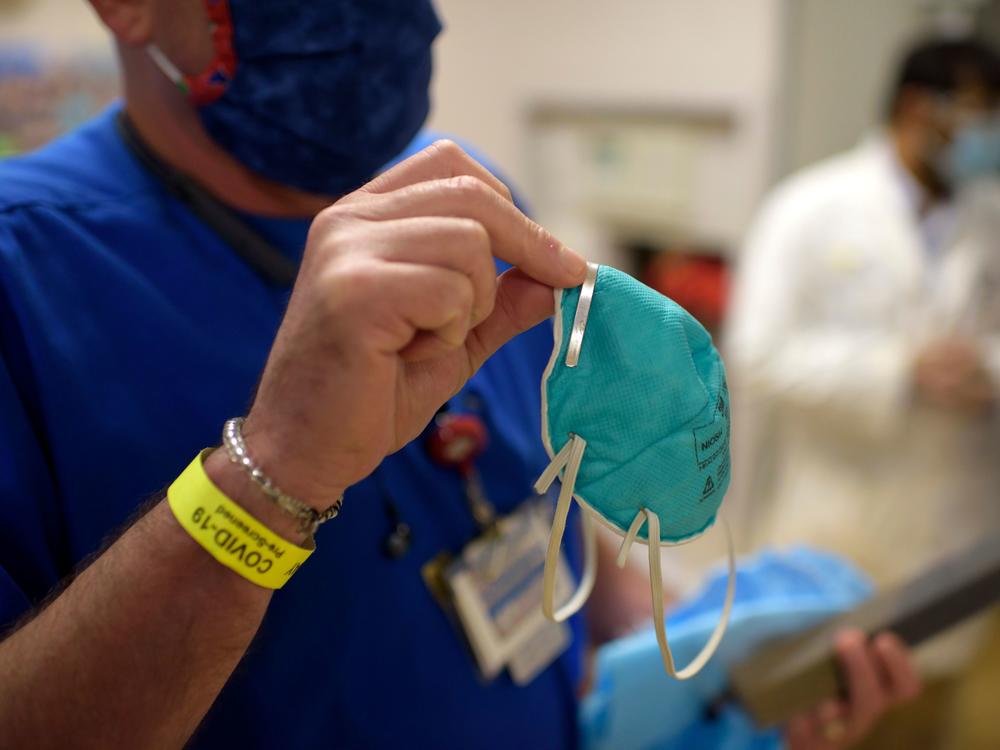
Why N95 Masks Are Still In Short Supply In The U.S.
Primary Content
A year ago, hundreds of desperate consumers were emailing Mike Bowen's Texas medical supply factory every day, looking to buy N95 medical respirator masks that can filter viruses: "Scared Americans and moms and old people and people saying, 'Help me,' " Bowen recalls.
Today, most consumers still aren't able to buy N95 masks, because the supply available to retailers remains very limited. Even hospital workers are still being asked to ration and reuse their supplies of N95s, and the website of the Centers for Disease Control and Prevention says, "N-95 respirators should not be used [by the general public] because they should be conserved for healthcare personnel."
Meanwhile, consumer demand for N95s and medical-grade, surgical-style masks keeps growing as the Biden administration emphasizes the use of masks by the public to slow the spread of the coronavirus — especially as new variants of it spread rapidly around the world.
From the start of the coronavirus pandemic, Bowen's company, Prestige Ameritech, and most other makers and distributors have prioritized supplying health care workers, who say they still don't have enough masks and other personal protective equipment.
The Biden administration has invoked the Defense Production Act to prioritize production of N95s and other medical supplies. But even with those measures, U.S. hospitals remain worried about their supply of these medical masks — more formally called respirators — despite efforts by factories to churn out billions more.
The story of N95 production over the last year in many ways reflects shortages seen throughout the U.S. medical supply during the pandemic — from ventilators and exam gloves to syringes and vaccines. The demand is global and sustained, putting pressure on a fragile supply chain that remains stressed and unable to keep up.
"Global demand continues to outpace production," says Nancy Foster, vice president of quality and patient safety at the American Hospital Association. Availability of N95 masks has improved since last spring, Foster says, but "we are continuing to use conservation measures within hospitals to protect the supplies we have, to extend the wear of N95s designed for one-time use." That includes asking hospital workers to wear each mask longer.
Costs for N95s — and other medical supplies, like medical gloves and gowns — have at least doubled. The use of N95s has increased 500% since July, according to Premier, a company that buys medical supplies on behalf of about 40% of U.S. hospitals.
"In most of the hospitals, nurses are wearing their N95s for five shifts," or up to 60 hours, says Mary Turner, president of the Minnesota Nurses Association and an intensive care nurse working with COVID-19 patients. "It's becoming the norm to not wear N95s the way they're supposed to be used."
A November survey by National Nurses United found the lack of protective gear like N95s remains a huge safety concern for its members. More than 80% of nurses reported reusing single-use items like N95 respirators, and about 20% of hospitals had recently limited the use of N95s.
Before the pandemic, there was little consumer demand for these products. Purchasers included people with compromised immune systems or others working in wildfire areas or on dusty home improvement projects.
That has changed. Everyone — from front-line grocery workers to travelers to teachers to people visiting vulnerable family members — is looking for the specialized masks.
N95s are the gold standard in masks because unlike cloth, surgical and KN95 alternatives, they're tested and approved by a federal agency as having demonstrated that "they can filter out a minimum of 95% of airborne particles under worst case test conditions," according to the CDC.
Nonetheless, N95s are still rarely available to consumers.
Shepard Medical Products, an Illinois-based company that sells supplies to drugstores and other retailers, hasn't sold a single N95 since March of last year. That's when makers of N95s called the company's president, Chris Humbert, to tell him, " 'We're done — we won't have any more product available for 2020.' "
So far this year, Humbert says, that shortage hasn't yet eased. Some wholesalers large enough to order directly from factories in China occasionally can get N95s to sell at hardware stores, for example, but "it's still very fragmented." The priority, he says, has been to supply health care facilities and government agencies. "I stopped trying, until hospitals are covered."
Fraud is also a major concern. Everyone, from nurses, hospitals, manufacturers and distributors, says vetting fake suppliers or identifying copycat N95 masks has been a huge concern.
Humbert says many new upstarts tried to sell him products billed as N95s, but because he couldn't verify their quality or efficacy, he decided it would be safer to remain out of stock.
"We didn't like being out of stock and disappointing any of our customers by not being able to supply, but we did not feel that we had a reliable source that could provide those products for us on par with the product that we had in place," Humbert says.
Exactly when American consumers might once again gain broader access to N95s depends on a lot of factors.
"I think if the vaccine rolls out faster, you're going to be able to get N95s faster," as the risks diminish and fewer people need N95s, says Kaitlin Wowak, a supply chain expert and assistant professor at the University of Notre Dame. (Public health workers urge even those immunized to continue pandemic precautions — including consistent mask-wearing — for now, until the pandemic is tamed.)
Broader availability of N95s also depends on manufacturing speed, Wowak notes, and on when backlogged orders from hospitals and other medical facilities can be filled.
The Biden administration has touted its plans to use the Defense Production Act to stimulate production. Wowak says that might mean manufacturers get more federal help finding the raw materials needed or coordinating distribution of supply. But it won't address some of the main challenges that affect the speed of manufacturing.
Wowak says how fast products like N95s are made is determined by three primary factors: the complexity of the equipment used to make the product, the availability of raw materials and the availability of trained workers.
Making vats of hand sanitizer at a rum distillery, in other words, is very different from ramping up an N95 factory, because of the cost and complexity.
Managing those costs and complexities has made the past year extremely busy for Mike Bowen, the co-owner of Prestige Ameritech. He and his partner started the company in 2005; it is one of the few makers of N95s based in the United States. Demand overwhelmed his factory a year ago when China stopped exporting the masks that most U.S. hospitals relied on for most of their supply.
"I've gotten requests for maybe a billion and a half masks if you add it up," Bowen told NPR in late February of last year. At the time, Bowen's company could produce 75,000 N95s a month.
He was troubled by the influx of orders, he said. They put him in a bind.
To make more N95s, Bowen would need new mask machines, each of which takes four months to custom build and costs as much as $1 million. To justify building extra machines, he needed assurance that U.S. hospitals and government agencies wouldn't just go back to buying cheaper Chinese-made masks once the pandemic was over.
He'd been burned before. A decade earlier, during the H1N1 flu pandemic, Prestige had made what Bowen called "the mistake" of investing in new machines and ramping up production for a need that dried up as suddenly as it began.
"One day — and it is literally one day — it just quits," Bowen told NPR last spring. "The demand is over."
He eventually did decide to expand last spring, as the COVID-19 pandemic worsened.
Bowen asked U.S. hospitals to sign multiyear deals for N95s. That gave him the funds to build nine new N95 machines, some of which are still coming online. The factory now makes 80 times more masks than it did a year ago.
"We're now selling 6 million [a month], and we have another 4 million coming on board," he says.
For the first time in a very long time, Bowen says, he has some excess supply he could start selling into the consumer market.
Copyright 2021 NPR. To see more, visit https://www.npr.org.