Section Branding
Header Content
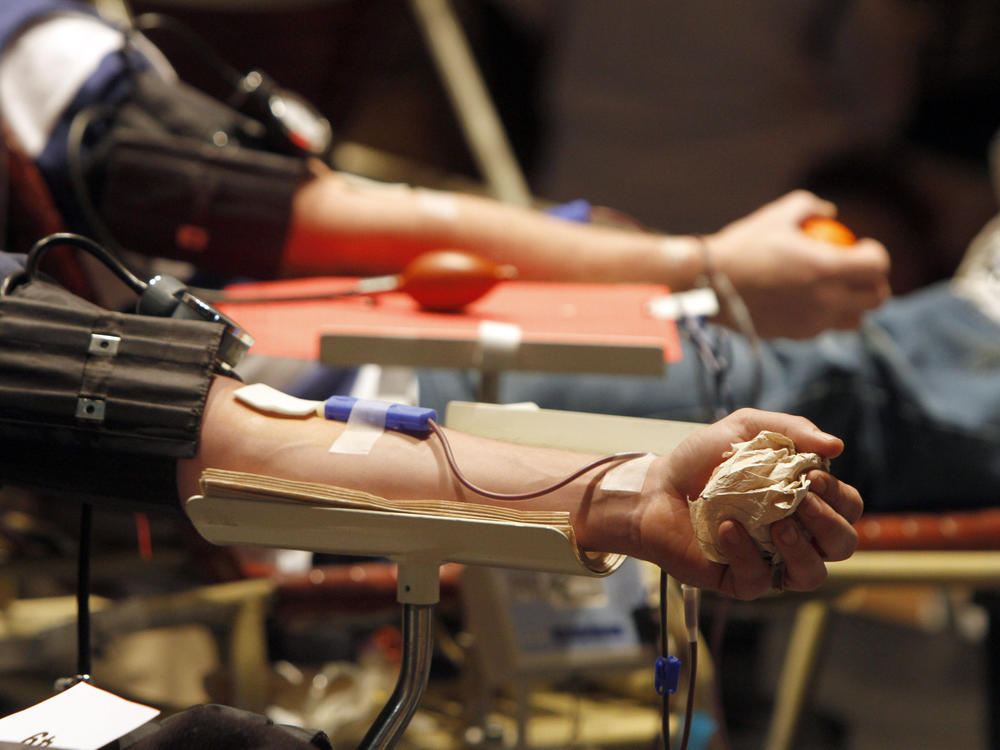
FDA moves to ease restrictions on blood donations for men who have sex with men
Primary Content
Updated January 27, 2023 at 11:05 AM ET
Updated 12:55 p.m. ET
The U.S. Food and Drug Administration issued proposed guidance Friday to ease restrictions on blood donations by men who have sex with men.
The change is expected to take effect after a public comment period.
The restrictions on donating blood date back to the early days of the AIDS epidemic and were designed to protect the blood supply from HIV. Originally, gay and bisexual men were completely prohibited from donating blood. Over time, the FDA relaxed the lifetime ban, but still kept in place some limits.
Under the current policy — last updated in 2020 — men who have sex with men can donate blood if they haven't had sexual contact with other men for three months.
The new proposed policy would eliminate the time-based restrictions on men who have sex with men (and their female partners) and instead screen potential donors' eligibility based on a series of questions that assess their HIV risk, regardless of gender. Anyone taking medications to treat or prevent HIV, including PrEP, would not be eligible.
The risk assessment would include questions about anal sex. Potential donors who've had anal sex in the last three months with a new sexual partner or more than one sexual partner would not be eligible to give blood.
The changes are aimed at addressing criticism that the current policy is discriminatory and outdated, as well as one more barrier to bolstering the nation's blood supply. Blood banks already routinely screen donated blood for HIV.
"We are moving now to an inclusive policy for blood donation," said Dr. Peter Marks, who leads the Center for Biologics Evaluation and Research at the FDA during a briefing Friday.
"We will continue to work to make sure that we have policies that allow everyone who wants to donate blood to be able to donate blood within what the science allows to make sure that the blood supply remains safe."
In crafting the new guidance, the FDA has been looking to the results of a study of about 1,600 gay and bisexual men to develop screening questions that can identify potential donors who are most likely to be infected with HIV.
Reaction to the news from advocates, medical groups and blood banks has been positive.
"The blood community is very excited about the proposed changes," says Kate Fry, CEO of America's Blood Centers. "We have advocated for a decade now for a move to an individual risk assessment model. So this is very welcome by blood centers across the country."
She stressed that all donated blood is carefully screened for HIV and that testing has improved dramatically to ensure the safety of the blood supply.
For many years, the American Medical Association, the American Red Cross and LGBTQ+ advocacy groups have pushed for a change to the federal rules on blood donations.
"These changes are 40-plus years in the making and they're a tremendous leap forward in elevating science over stigma," says Tony Morrison, a spokesperson for the advocacy group GLAAD.
But GLAAD and other groups say the changes still don't go far enough. They argue that some of the remaining restrictions are still unnecessary and stigmatizing, such as the prohibition against donations by people taking medication PrEP to prevent HIV.
"When we limit and defer people who are being proactive in their sexual health that stigmatizes them. The misconception is that people on PrEP are promiscuous or have a higher risk of HIV infection — that's categorically false," says Morrison.
So his group will continue to lobby the FDA to further ease restrictions.
The proposed changes in the blood donation rules will be open for public comment for 60 days. The FDA will then review those comments and issue a final rule, probably later this year. So monogamous gay men could start donating blood again sometime in 2023.
Copyright 2023 NPR. To see more, visit https://www.npr.org.