Section Branding
Header Content
Scientists restore brain cells impaired by a rare genetic disorder
Primary Content
Scientists have found a way to restore brain cells impaired by a rare and life-threatening genetic disorder called Timothy syndrome.
A type of drug known as an antisense oligonucleotide allowed clusters of human neurons to develop normally even though they carried the mutation responsible for Timothy syndrome, a team reports in the journal Nature.
The approach may help researchers develop treatments for other genetic conditions, including some that cause schizophrenia, epilepsy, ADHD, and autism spectrum disorder.
"It's immensely exciting because we now have the tools," says Dr. Sergiu Pasca, a professor of psychiatry and behavioral sciences at Stanford University and the study's senior author.
"It's the beginning of a new era for many of these diseases that we first thought were untreatable," says Dr. Huda Zoghbi, a professor at Baylor College of Medicine who was not involved in the research.
But most of these conditions involve multiple genes, not just one — and scientists don't yet know enough about these multiple gene disorders to effectively treat them with antisense oligonucleotides, Zoghbi says.
Insights from a rare disorder
Timothy Syndrome has been diagnosed in fewer than 100 people worldwide. Children born with it often have heart problems, autism, epilepsy, developmental delay, and intellectual disability.
But because Timothy syndrome is caused by a mutation in a single gene, it offers scientists a way to study changes that affect brain development.
"Rare syndromes that are very clearly defined genetically are sort of like windows, or Rosetta Stones, into understanding other, more common conditions," Pasca says.
So Pasca has spent the past 15 years learning how the mutation responsible for Timothy syndrome alters brain cells.
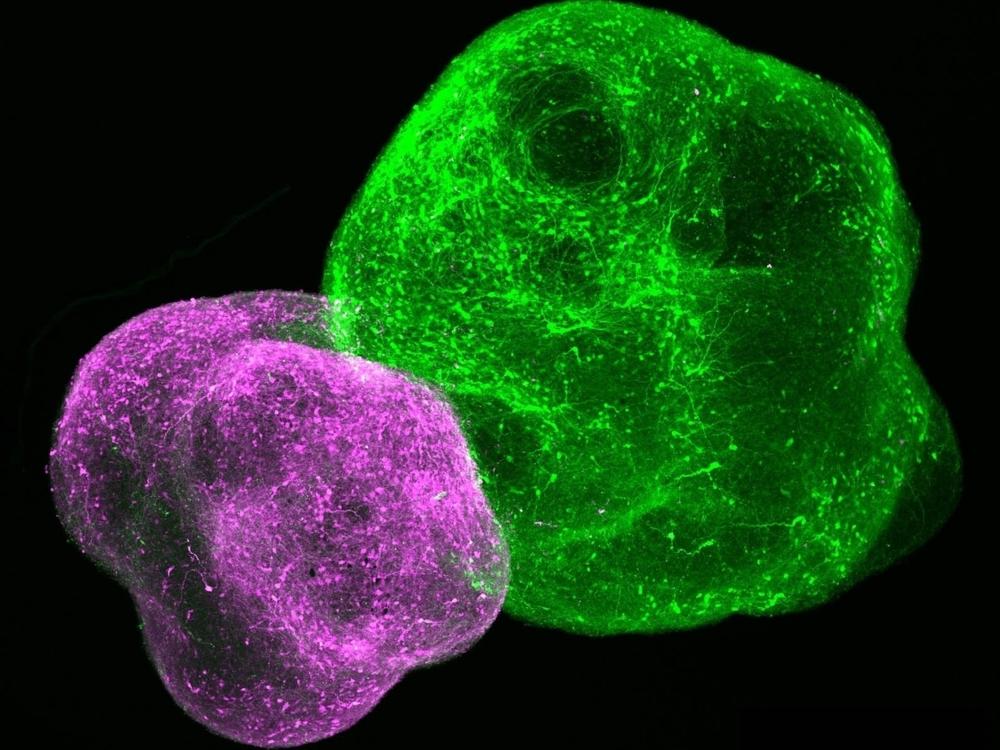
First, he and his team used skin cells from Timothy syndrome patients to grow neurons in a dish that carried the mutation. Then the team moved on to studying the mutation in brain organoids — living clusters of human neurons that assemble themselves into structures that resemble specific types of brain tissue.
Next, Pasca's team created brain "assembloids," which involve several organoids that form connections and interact, much the way areas of a developing brain do.
And in 2022, the team transplanted human organoids with the Timothy syndrome mutation into the brains of newborn rats. This allowed the human cells to keep developing much longer than they would have in a dish.
Repairing each cell
All of these experiments allowed Pasca's team to acquire a detailed understanding of how Timothy syndrome affects brain cells.
The mutation occurs on a gene called CACNA1C, which is involved in controlling the flow of calcium ions in and out of cells. This "calcium signaling," in turn, controls many of the processes a cell needs to function.
Pasca's lab showed that neurons with the Timothy syndrome mutation stayed abnormally small, and were less able to form connections. Certain mutated neurons also had an impaired ability to migrate from one area of the brain to another during development.
"We've essentially cataloged all these abnormalities," Pasca says. "And at one point, we just gathered enough information about the disease that a therapeutic approach just became self evident."
The approach meant developing an antisense nucleotide, a small piece of synthetic genetic material that alters the proteins made by a cell. The antisense nucleotide for Timothy syndrome was designed to replace a defective protein with a healthy version — in effect counteracting the mutation responsible for the disorder.
To see if the antisense drug worked, Pasca's team did an experiment with newborn rats. First, they transplanted brain organoids containing the Timothy syndrome mutation into the cerebral cortex of rats.
As the organoids grew, they began to develop the same defects seen in the brains of people with Timothy.
Then, the team injected the antisense drug into the rats' nervous systems.
"Within a couple of days, you start rescuing or restoring all those defects that we've observed over the years," Pasca says.
Neurons in the organoids became larger and formed more connections. The cells also migrated normally and had electrical activity indicating that the calcium signaling system was working properly.
From rats to people?
Pasca's lab hopes to try the antisense drug in people with Timothy syndrome in the next couple of years.
It is also studying how calcium signaling — the cellular process affected in Timothy syndrome — may play a role in much more common conditions, including schizophrenia, bipolar disorder, and autism spectrum disorder.
Meanwhile, scientists are working on antisense drugs for other rare genetic conditions that affect brain development. These include Angelman syndrome and Dravet syndrome.
An antisense drug for spinal muscular atrophy, a genetic disease that affects muscle strength, was approved by the Food and Drug Administration in 2016.
All of those conditions are caused by mutations to a single gene. Antisense treatments for conditions that involve multiple genes – like most forms of autism, schizophrenia, and epilepsy — are likely to be much harder to develop, Zoghbi says.
Even so, she says, there is now reason to believe that scientists are closing in on strategies to treat these diseases.
In 1985, Zoghbi left her practice as a child neurologist to do research because "we could offer nothing" to patients with devastating genetic disorders like Rett syndrome and spinocerebellar ataxia. "We didn't know what caused the diseases," she says.
Now, scientists know the genetic changes responsible for hundreds of childhood conditions, and they are beginning to develop treatments for some, including Timothy syndrome.
"That's a dream come true for me," Zoghbi says.