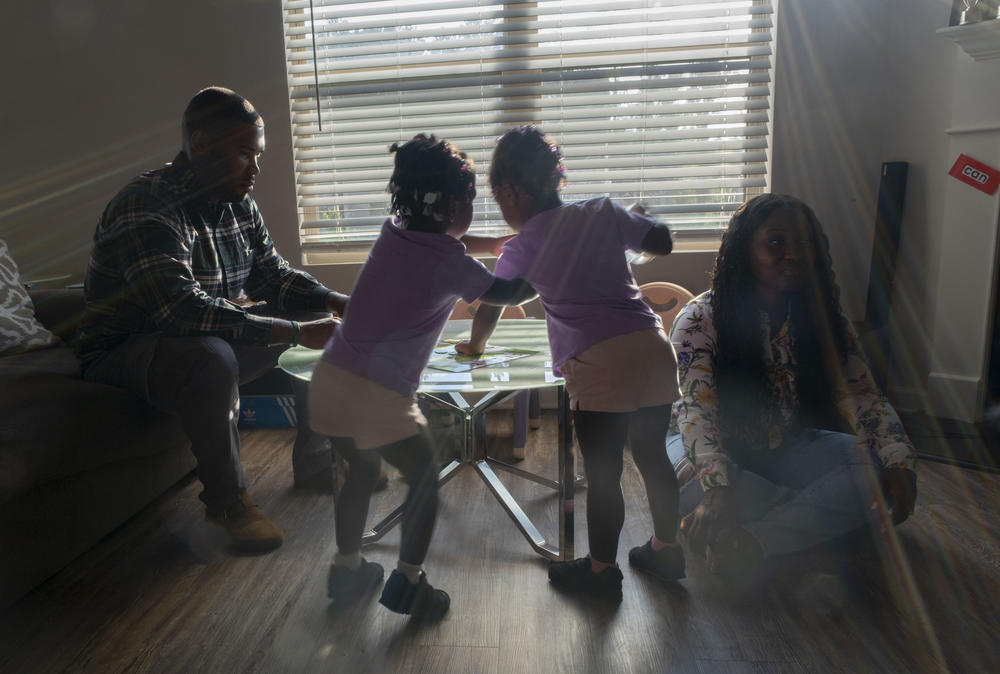
Caption
Justice Altidor and her twin sister, Journey, center, play in between their parents at home.
Credit: Allexa Ceballos/GPB News
|Updated: August 30, 2024 1:34 PM
LISTEN: Four children might not be breathing on their own if not for innovative technology from Georgia Tech and pediatric cardiologists at Children’s Healthcare of Atlanta. But the support is not yet approved by the U.S. Food and Drug Administration. And Atlanta is one of a handful of places nationwide to offer infants the surgery. GPB’s Ellen Eldridge has more.
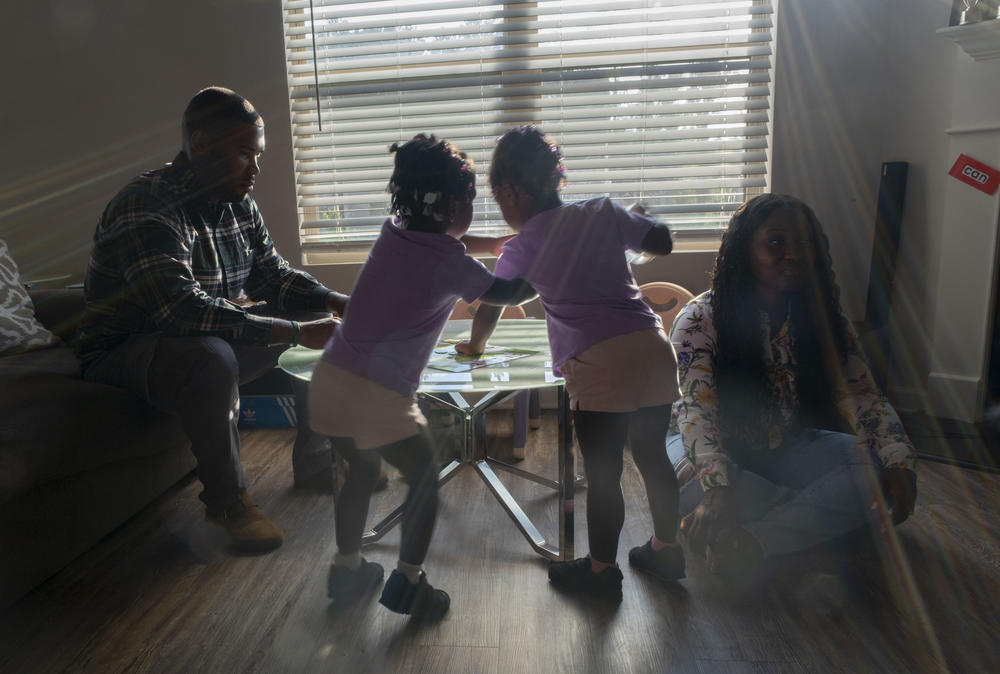
Justice Altidor and her twin sister, Journey, center, play in between their parents at home.
Justice Altidor and her twin sister, Journey, speak their own language.
The 4-year-old girls might not have been speaking at all without an experimental surgery to keep Justice’s airway from collapsing when she was an infant.
Their mother, Emmanuella Altidor, says she knew Justice had a heart condition before she gave birth to the twins.
"They knew she had the double aortic arch," she says. "They just didn't know how severe it was until she was born. And then they were on standby, by the grace of God, to take her, whisk her away."
Because of the advanced knowledge of Justice’s heart issue, doctors rushed her to the neonatal intensive care unit before mom could even say hello and hold her baby.
"After I did recovery, I was able to see her, and she was incubated," Altidor says. "And she stayed that way all the way until she got the surgery, four months later."
Dr. Kevin Maher is a pediatric cardiologist with Children’s Healthcare of Atlanta.
He says infant heart issues also often come with weak tracheas, airways not strong enough for babies to breathe.
Maher says, in his hospital, these are some of the most common issues they see in neonatal intensive care, but there aren’t great treatments available.
"We've had these kids that can spend, you know, their entire life with a breathing tube," he says. "And the moment you would take out those breathing tube, the airways would collapse and they would go immediately go into respiratory arrest."
Now, a team of Georgia Tech engineers have a custom 3-D printer to splint and support the newborn's airway.
Think of it as a cast of the baby’s own trachea.

An Airway Support Device like this supports the esophagus, keeping it open as it does the work of a trachea. The device is made of biodegradable material that is naturally and safely absorbed in the body.
Children’s is one of only five hospitals in the nation offering the surgery, and while the procedure waits for full FDA approval, every single surgery has to wait for its own go ahead from the agency.
So far, Justice is the fourth patient at Children’s approved for the supportive device.
The medical team submitted Justice’s case to the FDA for approval, which they got in October 2020 amid the COVID-19 pandemic.
"We were quarantined in the hospital for the most part," she says. "They were allowing me to go back and forth because I did have Journey still here and needed to care for her as well."
Justice could eat and breathe on her own just a few weeks after surgery, and she was discharged from the hospital.
The Altidor family celebrated the twins’ fourth birthday this summer in the Bahamas.
The family is home now, preparing for the back-to-school season with a math skills board game.

The Altidor family poses for a portrait.

Justice Altidor and her twin sister, Journey, center, play in between their parents at home.

Justice Altidor, center, and her twin sister, Journey play a game with their mom and dad.

Journey, left, and Justice, right, play together at home.

Justice is one of four children who might not be breathing on their own if not for innovative technology from Georgia Tech and pediatric cardiologists at Children’s Healthcare of Atlanta.

Emmanuella, Justice and Journey play a board game together at home.

Justice and Journey's father plays a board game with his twin daughters at home.

Justice, center, points to a number on the board game in front of her.

Justice Altidor sits in her mother Emmanuella's lap as she speaks with GPB's Ellen Eldridge.

Emmanuella Altidor sits to play with her twin daughters at home.

Emmanuella laughs as her daughter Justice plays a board game. The family is home now, preparing for the back-to-school season with a game focused on math skills.













There is no "after" surgery. The device just dissolves in the throat over time, and, aside from the scars, all Justice knows about the surgery are the stories she hears.
"After she first came out of surgery, you could feel the plastic in her chest," Altidor says. "Now it's just a flat surface. You wouldn't even know that she had something done."
Altidor says she can only imagine what Justice’s life might have been without the innovative device.
Dr. Kevin Maher says a fifth use of the 3D airway at Children’s is being planned, pending the OK of the surgery from the FDA.