Section Branding
Header Content
Chinese Pharmaceutical Makers Seek Approval For New Coronavirus Vaccines
Primary Content
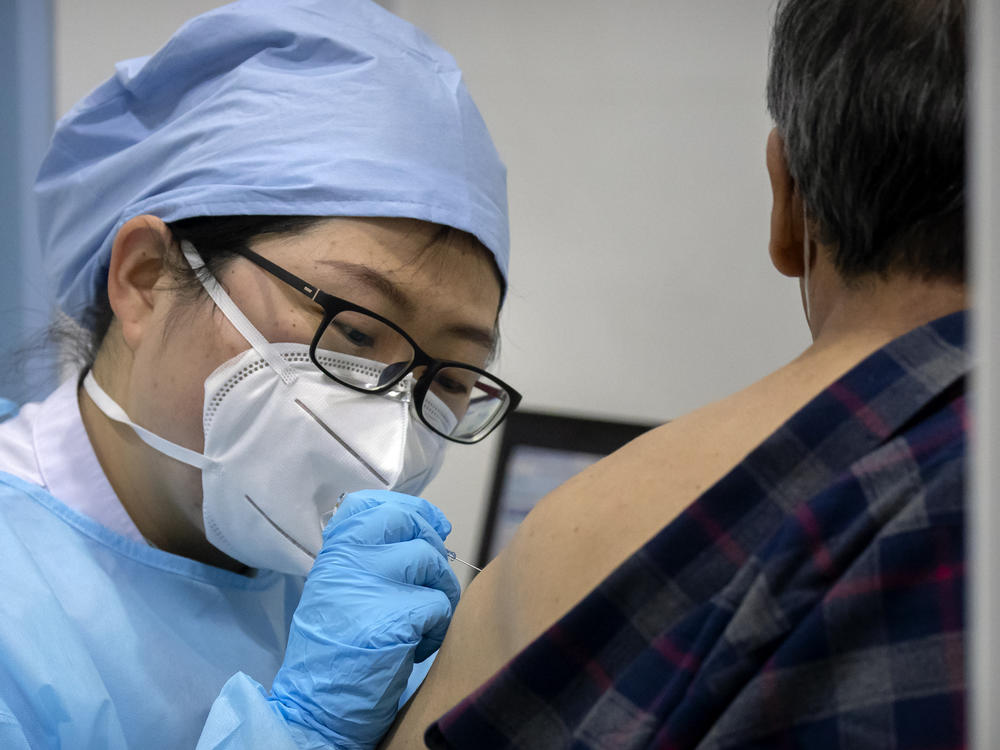
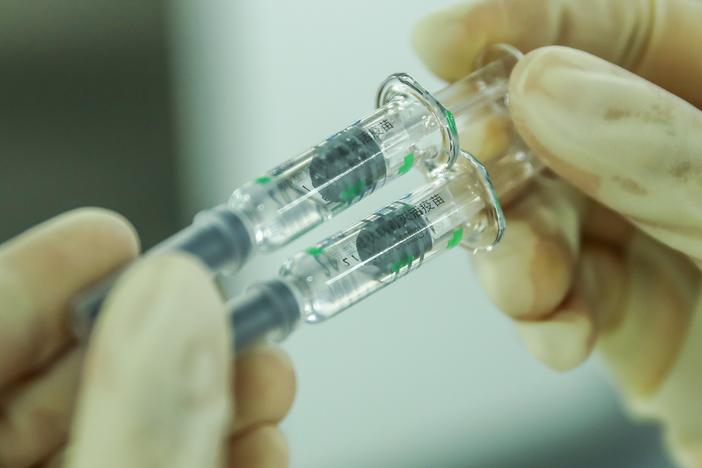
Chinese pharmaceutical makers are seeking market approval from Beijing for two new coronavirus vaccines – one that has shown 72% efficacy and another 69% efficacy in human Phase III trials.
The separate announcements on Wednesday come from Sinopharm for its second vaccine after the state-run company's first was approved for distribution in December, and from CanSino Biologics, Inc. (CanSinoBIO), for its first vaccine.
Another vaccine, produced by Beijing-based Sinovac, received market approval earlier this month, although it – like Sinopharm's first vaccine — had been in use since July on an emergency basis.
Unlike the revolutionary mRNA vaccines developed in the West by Moderna and Pfizer/BioNTech, which use a piece of the virus' genetic code to trigger an immune response, three of the four Chinese vaccines are based on a more conventional approach that uses an inactivated form of the coronavirus.
The fourth – the one made by CanSinoBIO – is a single-dose vaccine that is based around a technique called viral vector, which uses a modified version of a different virus as a vector to deliver instructions to a cell. China is also reportedly working on its own mRNA vaccine.
One advantage for the Chinese vaccines already approved or seeking market approval is that they can be stored at room temperature, while the Moderna vaccine must be stored at -20 degrees Celsius (-4 degrees Fahrenheit) and the Pfizer vaccine at -70 C (-94 F), nearly as cold as dry ice.
China has yet to approve any Western vaccines, preferring to rely on its own. However, Hong Kong's government has agreed to buy 7.5 million doses each from Sinovac; the British-Swedish firm AstraZeneca; and Fosun Pharma, which is delivering the Pfizer/BioNTech vaccine.
After delays, the first 1 million doses of the Sinovac vaccine arrived in Hong Kong last week. As of Wednesday, the first shipment of the Pfizer vaccine had yet to arrive, but was expected within days, according to the South China Morning Post.
Despite moving ahead on new vaccines, China, where the virus that causes COVID-19 was first identified more than a year ago, has been slow in getting the country's 1.4 billion people inoculated against the disease. It vaccinated tens of thousands of people on the first day of its official drive in early January. Since then it has inoculated large numbers of people, but still a relatively small portion of its vast population.
Health authorities say that about 40 million doses of the two-dose vaccines have been administered so far, covering a first shot for only about 3% of the population, NPR's Beijing correspondent Emily Feng reported Wednesday. That's well short of the minimum 700 million people that the chairman of the China National Biotec Group (CNBG), Yang Xiaoming, told state-run media in December would be required to adequately protect the country from COVID-19.
China is prioritizing workers in the health care, transportation and shipping sectors for the first round of vaccinations. But unlike most other countries, it isn't immediately focusing on seniors. Instead, people aged 18-59 who are considered at high risk and highly likely to spread the virus are being targeted, Wang Bin, an official of National Health Commission, told reporters in January.
As the vaccine supply increases, people 60 years and older will gradually be phased in, Wang said.
That has reportedly caused concern among some older Chinese.
Instead, China seems to be banking on its current low positivity rate, which has been bolstered by extensive testing, quarantines and travel restrictions, to keep SARS-CoV-2 at bay within its own borders.
Meanwhile, despite its lagging vaccine drive, Beijing has been sending millions of doses abroad. Many developing countries are counting on Chinese-developed vaccines as affordable and available options, and China's foreign ministry on Feb. 8 said it will provide them to 53 countries.
Serbia and Hungary are among the recipients, as are several countries in Latin America. Thailand on Wednesday received its first 200,000 of 2 million doses it expects to receive of the Sinovac vaccine.
Copyright 2021 NPR. To see more, visit https://www.npr.org.
Bottom Content