Section Branding
Header Content
WHO greenlights the world's first malaria vaccine — but it's not a perfect shot
Primary Content
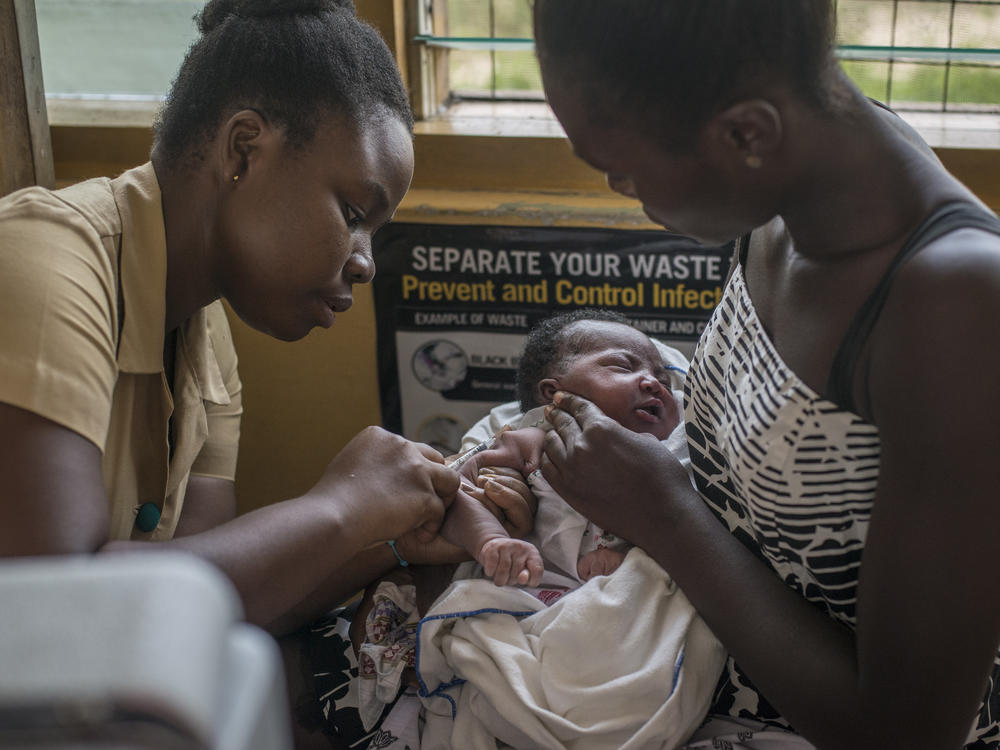
The world's arsenal against malaria just got a fancy new bazooka. But it's not the easiest weapon to deploy, it only hits its target 30 to 40% of the time, and it's not yet clear who's going to pay for it.
The weapon in question is the RTS,S vaccine from GlaxoSmithKline, which on Wednesday got the green light from the World Health Organization for widespread use.
This is not only the first authorized malaria vaccine, it's also the first vaccine ever approved for use against a parasitic disease in humans.
The recommendation comes after RTS,S showed positive results in a pilot program in Ghana, Kenya and Malawi. The vaccine cut malaria cases by 40% and reduced hospitalizations of the potentially deadly disease by nearly a third.
Tedros Adhanom Ghebreyesus, WHO's director general, called the approval of RTS,S a historic moment.
"The long-awaited malaria vaccine for children is a breakthrough for science, child health and malaria control," he said.
RTS,S won regulatory approval from the European Medicines Agency back in 2015 but WHO wanted to wait for the results of this latest pilot program before recommending it for use in countries with moderate to high levels of malaria transmission. The expectation is that it will be used primarily in sub-Saharan Africa, where the mosquito-borne disease is one of the top killers of children, claiming nearly a quarter of a million lives each year.
"This opens up a whole new avenue for malaria control," says David Schellenberg of WHO's Global Malaria Program, who says that RTS,S gives health officials a new powerful tool to fight the disease.
Combined with bed nets, spraying for mosquitoes and new drugs, the vaccine could have a major impact in places where malaria remains a chronic problem, Schellenberg says.
Matshidiso Moeti, WHO regional director for Africa, says she's delighted by the new recommendation.
"For centuries, malaria has stalked sub-Saharan Africa, causing immense personal suffering," Moeti said during the announcement. Nearly 95% of all malaria cases globally occur in Africa.
"Now for the first time ever, we have a [malaria] vaccine recommended for widespread use. Today's recommendation, therefore, offers a glimmer of hope for the continent," she said.
But the vaccine won't be rolling out across Africa tomorrow. It's still unclear where the money to purchase doses will come from. Also it's a complicated vaccination to administer, requiring four injections spread out over the first two years of a child's life. And given that it only prevents malaria 30 to 40% of the time, this vaccine is far less effective than health officials had hoped.
Pedro Alonso, head of WHO's Global Malaria Program, says part of the problem is that malaria is a complicated disease. "This is a parasitic disease," he points out. The parasite life cycle plays out in multiple stages in different parts of the human body and in the mosquito hosts. "This is orders of magnitude more complex in terms of the biology of the causative organism [than a virus]," he says.
Decades of research have gone into developing RTS,S. Alonso would love to see a vaccine that's 95% effective in preventing malaria but says the scientific community is still a long way off from developing that: "But what we do have right now is a vaccine that can be deployed, that is accepted, that is safe and that can have a massive impact in terms of lives saved and episodes of malaria averted."
Countries that decide to move forward with administering RTS,S still need to figure out how to pay for it and how to integrate it into their childhood immunization schedules. GlaxoSmithKline had donated 10 million doses of the vaccine for pilot programs and has now pledged to deliver 15 million doses a year at a price of 5% above cost. Eventually GSK says it plans to transfer production to a producer in India.
Copyright 2021 NPR. To see more, visit https://www.npr.org.