
Caption
Researchers will enroll a total of 56 healthy, HIV-negative adult volunteers across all four sites involved with the first HIV vaccine trial in humans..
Credit: Anna Shvets / Pexels
Emory University is participating in Phase I clinical trials to evaluate the use of messenger RNA technology in HIV vaccines. GPB’s Ellen Eldridge has more.

Researchers will enroll a total of 56 healthy, HIV-negative adult volunteers across all four sites involved with the first HIV vaccine trial in humans..
Researchers with Emory’s Hope Clinic and Vaccine Center will determine whether immunogens, which are molecules capable of eliciting an immune response, can induce specific B-cell responses that have the potential to lead to broadly neutralizing antibody development.
The ultimate goal is to vaccinate people against human immunodeficiency virus — HIV, the virus that causes AIDS, acquired immunodeficiency syndrome.
AIDS is a chronic, potentially life-threatening condition that damages a person's immune system by interfering with the body's ability to fight infection and disease.
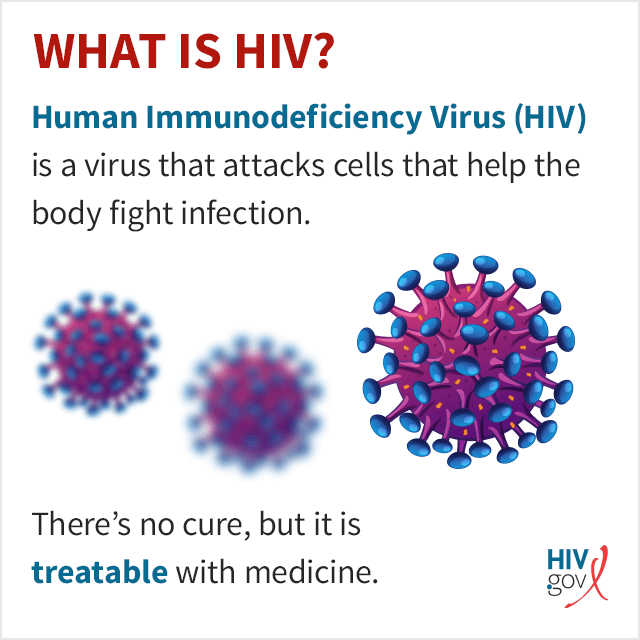
HIV specifically attacks helper T cells. Without an adequate supply of helper T cells, the immune system cannot signal B cells to produce antibodies or cytotoxic T cells to kill infected cells.
Moderna developed its HIV vaccine using the same technology used to create its COVID-19 vaccine. While these vaccines for COVID are newly available to the public, researchers have been studying and working with messenger RNA vaccines for decades.
The technology was being pursued as one of the platforms for HIV vaccine development before the COVID pandemic, but the vaccine wasn't ready for clinical trials at the time, Emory University Professor of Medicine Dr. Sri Edupuganti said.
MORE: Emory Conducts Moderna COVID-19 Vaccine Clinical Trials For Children 6 Months to 11 Years
Researchers are using reverse engineering to design the vaccine, Edupuganti said, "meaning you find the antibodies that work to kill the virus, and then you work your way backwards to see which part of the virus is that the best part then to use as a vaccine."
mRNA never enters the nucleus of the cell where our DNA (genetic material) is located, so it cannot change or influence our genes.
The biggest scientific challenge is discovering which part of the HIV virus will induce effective antibodies to fight, she said.
Many different parts of the virus have been tested as vaccine candidates, but none of them are able to induce effective antibodies to fight the virus, Edupuganti said. "So it's actually more of a scientific challenge than, really, anything else."
RELATED: 'It's An Honor' Says Local COVID-19 Vaccine Trial Participant
In Moderna's COVID vaccine, the spike protein is targeted. The mRNA enters muscle cells and instruct the cells’ machinery to produce a harmless piece of what is called the spike protein, according to the Centers for Disease Control and Prevention.
The spike protein is found on the surface of the virus that causes COVID-19. After the protein piece is made, cells break down the mRNA and remove it.
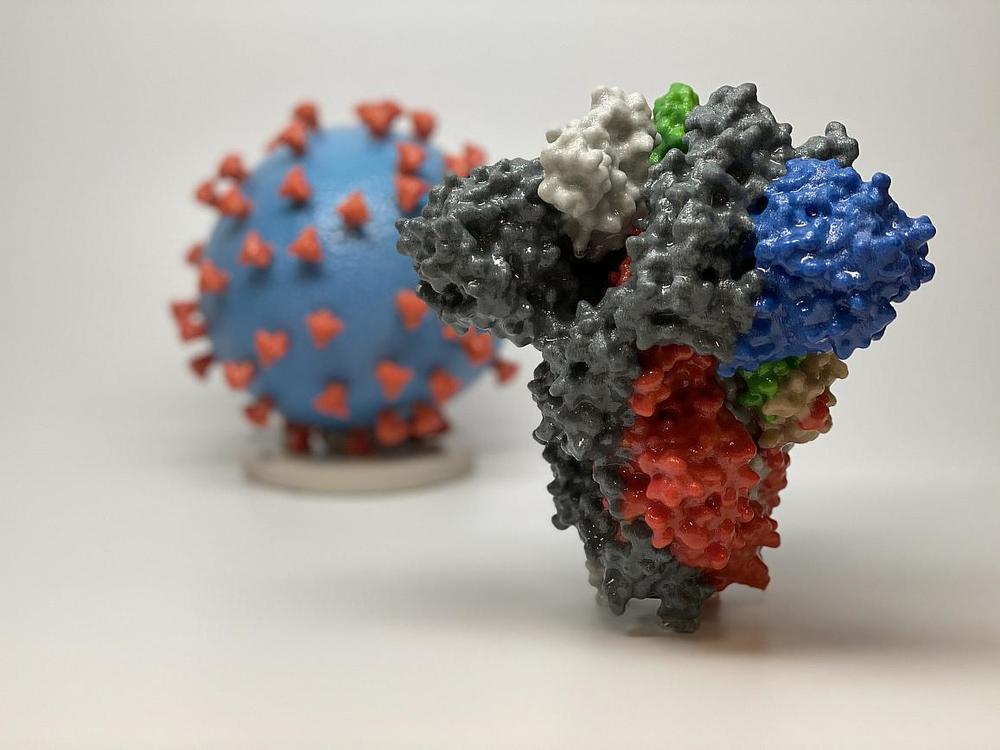
3D print of a spike protein of SARS-CoV-2—also known as 2019-nCoV, the virus that causes COVID-19—in front of a 3D print of a SARS-CoV-2 virus particle. The spike protein (foreground) enables the virus to enter and infect human cells. On the virus model, the virus surface (blue) is covered with spike proteins (red) that enable the virus to enter and infect human cells.
The Hope Clinic, which is a part of the Division of Infectious Disease at Emory School of Medicine, is one of four sites in the country participating in the study in partnership with the International AIDS Vaccine Initiative, a global not-for-profit, public-private partnership working to accelerate the development of vaccines to prevent HIV infection and AIDS.
The George Washington University School of Medicine and Health, Fred Hutchinson Cancer Research Center (Fred Hutch) in Seattle, and the University of Texas–Health Science Center at San Antonio are also sites for IAVI G002, the current clinical trial. Scripps Research and IAVI developed the vaccine candidates with support from the Bill & Melinda Gates Foundation, the Center for HIV/AIDS Vaccine Immunology and Immunogen Discovery (CHAVI-ID) at NIAID at the NIH and Moderna.
A previous clinical trial, IAVI G001, showed that an adjuvanted protein-based version of the priming immunogen eOD-GT8 60mer induced the desired B-cell response in 97% of recipients.
The study now recruiting, IAVI G002, will build upon these previous findings. Interested participants can sign up on the clinic's website here.