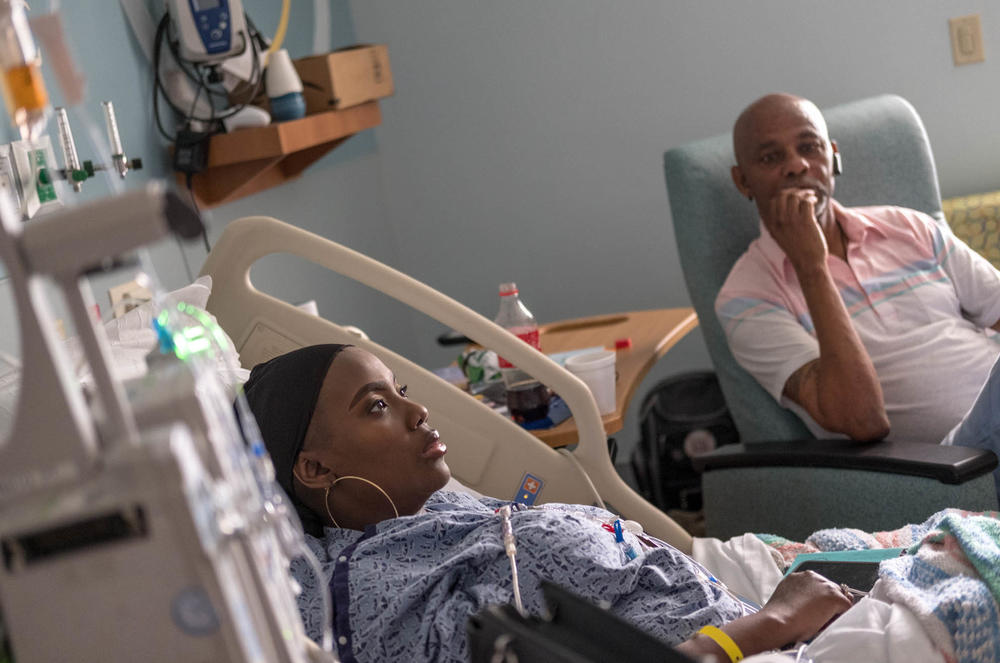Section Branding
Header Content
Sickle cell patient's success with gene editing raises hopes and questions
Primary Content
Victoria Gray was wandering through the British Museum in London last week when she spotted a small wooden cross hanging on the wall.
"It's nice seeing all the old artifacts, especially the cross," Gray said. "Religion is something that I hold close to my heart, and my faith is what brought me this far."
Almost four years ago, Gray became one of the first patients with a genetic disorder — and the first patient with sickle cell disease — to get an experimental treatment that uses the revolutionary gene-editing technique known as CRISPR.
Today, all of Gray's symptoms are gone, and she was in London last week to describe her landmark experience at the Third International Summit on Human Genome Editing. The summit brought together more than 400 scientists, doctors, patients, bioethicists and others from around the world to air the promise of gene editing as well as a host of thorny questions that the technology is raising.
"God did his part for what I prayed about for years," Gray said. "And together, hand in hand, God and science worked for me."
An NPR reporting team, which has had exclusive access to chronicle Gray's experience, spent the day with Gray before her appearance at the three-day summit.
"I'm excited," said Gray, who lives in Forest, Mississippi. "Nervous, but excited."
Throughout Gray's life before she got the treatment, the deformed, sickle-shaped red blood cells caused by the genetic disorder would regularly incapacitate her with intense, unpredictable attacks of pain. Those crises would send Gray rushing to the hospital for pain medication and blood transfusions. She could barely get out of bed many days; when she became a mom, she struggled to care for her four children and couldn't finish school or keep a job.
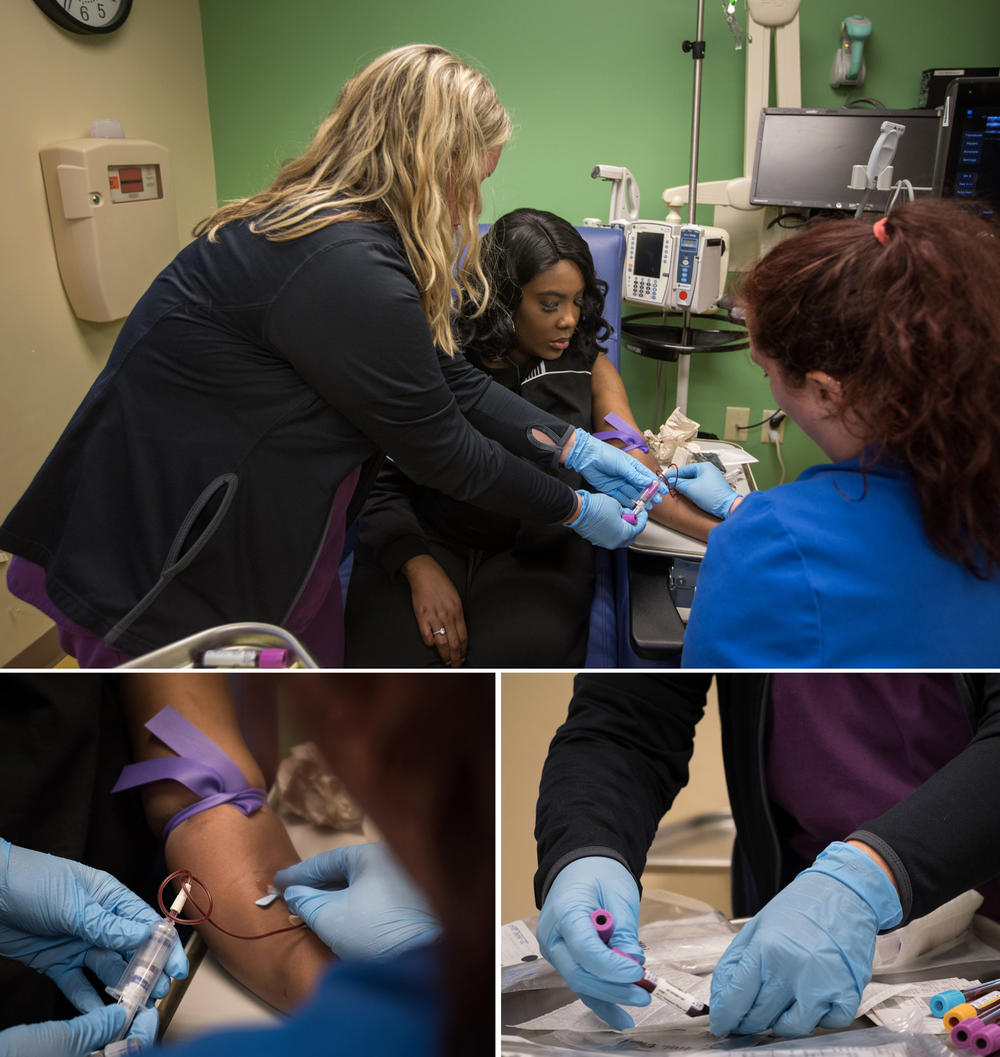
But then she received the treatment on July 2, 2019. Doctors removed some of her bone marrow cells, genetically modified them with CRISPR and infused billions of the modified cells back into her body. The genetic modification was designed to make the cells produce fetal hemoglobin, in the hopes the cells would compensate for the defective hemoglobin that causes the disease.
Gray, who's 37, now works full time as a Walmart cashier, is able to keep up with her teenagers and was eager to explore London on her first trip outside the United States. Though she hadn't slept much on the overnight flight, Gray couldn't wait to see the sights with her husband, Earl.
"I would never have been able to walk this long before," she said while sightseeing through Trafalgar Square. "It's a huge difference — night and day. I feel like I got a second chance."
After the museum, Gray and her husband headed to the London Eye, a huge Ferris wheel that towers over the city. Gray was keen for a ride, even though she's afraid of heights.
"It's a beautiful view," she said as they circled to the top and she saw Big Ben and other landmarks in the distance. "Part of my dreams coming true."
The next morning, Gray and her husband made their way through the crowd at the conference, held at the Francis Crick Institute, and found seats in the auditorium.
"Hello, everyone. I'm very pleased to see so many people here," said Robin Lovell-Badge, who led the summit.
Speaker after speaker described the latest scientific advances in gene editing.
"There are more than 200 patients to date, including Victoria, Patrick and Carlene pictured here, that have been treated in clinical trials with CRISPR nucleases targeting DNA sequences that, when disrupted, offer clinical benefit," David Liu told the crowd via a remote link.
Liu has developed new gene-editing techniques at the Broad Institute in Cambridge, Massachusetts. "You'll hear more from Victoria about her experience directly later today."
Finally, it was Gray's turn at the podium.
"Good evening. I'm Victoria Gray. And I'm a 37-year-old mother of four and a sickle cell survivor," she began. "Take a moment to go on a journey with me."
For 10 minutes, Gray repeatedly choked back tears as she described her life with sickle cell, including her children's fears that she would die. She detailed one especially tortuous pain crisis.
"During this hospital stay, with a ketamine infusion in one arm and a Dilaudid infusion in the next — but still no pain relief — I called all the doctors into the room and told them I could no longer live like this," Gray said. "I went home and continued to pray, and looked to God for answers."
Gray explained how she finally received the CRISPR gene-edited cells — "supercells," she calls them — as part of a study.
"The life that I once felt like I was only existing in, I am now thriving in," she told the assembled scientists, doctors, bioethicists and others. "I stand here before you today as proof that miracles still happen — and that God and science can coexist."
As Gray walked off the stage, the crowd gave her a standing ovation.
Vertex Pharmaceuticals and CRISPR Therapeutics, the companies that sponsored the study that Gray volunteered for, say they have now treated 75 patients who have sickle cell or the related condition beta thalassemia.
After the gene-editing treatment, 42 of 44 beta thalassemia patients were able to discontinue the transfusions that had been keeping them alive. And all 31 sickle cell patients were free of symptoms, even though all had been previously diagnosed with severe cases.
Based on those results, the companies are asking the Food and Drug Administration to approve the treatment for severe sickle cell and beta thalassemia. That approval could come as soon as this summer and would make it the first therapy created through this sort of gene editing to become widely available.
But for the rest of summit, speakers warned that there are still important questions about this treatment and other gene-editing therapies in the pipeline, including how long the benefits will last.
Also, the sickle cell treatment is expected to be very expensive — possibly costing millions of dollars. That raises questions about whether it will be available to the patients who need it the most, especially less affluent people in the U.S. and in countries where sickle cell is most common, such as those in sub-Saharan Africa.
"I worry that when gene editing comes to market for sickle cell, that the very states in the United States that won't expand Medicaid or access to insurance, which are some of the very states where prevalence is the highest, will inhibit the affordability and availability of the therapy," said Melissa Creary of the University of Michigan, who studies policy issues raised by sickle cell.
An estimated 1,000 babies are born every day worldwide with sickle cell. The disease affects an estimated 100,000 people in the U.S., many of whom are African American, along with an estimated 20 million people worldwide.
"The absolute central factor in the uptake of a new therapy is cost and accessibility. A new therapy can be extremely effective, and even a cure for sickle cell, but if it's not made accessible to the average patient, it won't be used," said Arafa Salim Said of the Sickle Cell Disease Patients Community of Tanzania.
In addition, the treatment is complicated, requiring a bone marrow transplant. Very few countries in sub-Saharan Africa currently have the resources to perform that procedure.
"I hope this will be available to everyone who needs it," Gray said after speaking and listening to the summit's other presentations. She has relatives who are still struggling with sickle cell. "It's horrible knowing that something is out there that can cure your disease but you can't access it."
Copyright 2023 NPR. To see more, visit https://www.npr.org.